IonChannels@IBGM
Group: Ion channels and vascular pathophysiology
Group members:
-
- Dr. José Ramón López López (PI) Full profesor, Biochemistry
- Dra. M Teresa Pérez García (PI) Full Professor, Physiology
- Dra Pilar Cidad Velasco (Postdoctoral researcher)
- Nuria Daghbouche Rubio (PhD student)
- Lucía Alonso Carbajo (PhD student)
- Inés Álvarez Miguel (PhD student)
- Marycarmen Arévalo Martínez (PhD Student)
- Sara Moreno Estar (PhD student)
- Esperanza Alonso Alonso (Lab technician)
Contact:
Dr M. Teresa Pérez García
Departamento de Fisiología
Edificio IBGM, c/ Sanz y Forés 3
47003 Valladolid
Tel 983 184590
tperez@ibgm.uva.es
Research interests:
The group has a long research activity in the field of the physiology and the molecular biology of ion channels. The current projects of the group represent a focused proposal to define the physiological and pathological roles of ion channels in vascular smooth muscle cells, using two different models of vascular disease, essential hypertension and intimal hyperplasia. In particular, we are interested in:-
Electrical remodeling of vascular smooth muscle cells in esential hypertension
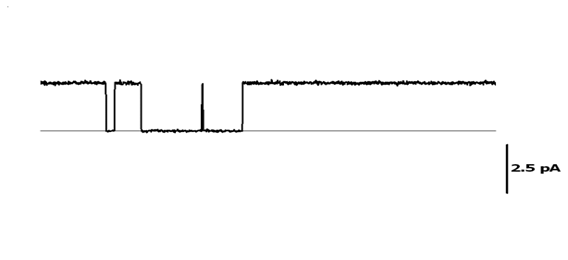
The search of new therapies for hypertension treatment requires the previous identification of the disease-specific expression profile, characterizing gene expression alterations and their functional 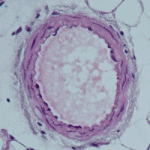 significance. We are employing genomic and proteomic approaches to determine the expression pattern of a large number of ion channels, identify differential display under pathological situations, and study their functional contribution both in isolated cells and whole vessels in order to establish correlations between changes in expression and functional alterations. Once the candidate genes are identified we study the effect of over-expression, gene silencing or immunopharmacological blockade on the hypertensive phenotype. This study will help to define emerging concepts and opportunities for the development of new vasoactive drugs, which may rely on targeting disease-specific changes in ion channel expression as a mechanism to lower vascular tone during hypertensive diseases.
significance. We are employing genomic and proteomic approaches to determine the expression pattern of a large number of ion channels, identify differential display under pathological situations, and study their functional contribution both in isolated cells and whole vessels in order to establish correlations between changes in expression and functional alterations. Once the candidate genes are identified we study the effect of over-expression, gene silencing or immunopharmacological blockade on the hypertensive phenotype. This study will help to define emerging concepts and opportunities for the development of new vasoactive drugs, which may rely on targeting disease-specific changes in ion channel expression as a mechanism to lower vascular tone during hypertensive diseases.
-
Ion channel as therapeutical targets for intimal hyperplasia
We have found that phenotypic modulation associates with an increased functional expression of a voltage-dependent potassium (Kv) channel, Kv1.3 channel. The blockade of this channel decreases human VSMC proliferation in vitro and neointima formation in vivo. The underliying mechanisms are far from being clear, but since there are many selective Kv1.3 blockers, some of them currently under clinical trials for other applications, our discoveries could facilitate the development of alternative, more efficient therapi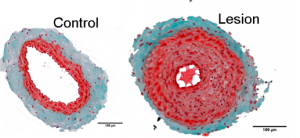 es to prevent restenosis. For this to occur, the following goals need to be achieved: 1) Characterization of the molecular and cellular mechanisms regulated by Kv channels and related to proliferation in model systems, both in vitro and in vivo. 2) Development of appropriate animal models and/or studies with
es to prevent restenosis. For this to occur, the following goals need to be achieved: 1) Characterization of the molecular and cellular mechanisms regulated by Kv channels and related to proliferation in model systems, both in vitro and in vivo. 2) Development of appropriate animal models and/or studies with 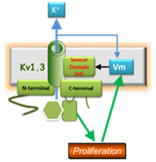 human
human  samples to validate these mechanism and test new therapies. 3) Coalescence of the information to allow the achievements of 1) to inform 2) and vice versa. This project will help to define the consequences of the modulation of the channels in the prevention and treatment of restenosis with the goal of developing new therapies.
samples to validate these mechanism and test new therapies. 3) Coalescence of the information to allow the achievements of 1) to inform 2) and vice versa. This project will help to define the consequences of the modulation of the channels in the prevention and treatment of restenosis with the goal of developing new therapies.
Selected publications
- Alvarez-Miguel I, Cidad P., Pérez-García MT and Lopez-Lopez JR and (2017) Differences in TRPC3 and TRPC6 channels assembly in mesenteric vascular smooth muscle cells in essential hypertension. J. Physiol 595(5):1497-1513)
- Jiménez-Pérez L, Cidad P, Alvarez-Miguel I, Santos-Hipólito A, Torres-Merino R, Alonso E, dela Fuente MA, Lopez-Lopez JR & Pérez-García MT (2016) Molecular determinants of Kv1.3 potassium channels-induced proliferation. J Biol Chem 291:3569-80.
- Turczyńska KM, Swärd K, Tran TH, Wohlfahrt J, Mattisson IY, Ekman M, Nilsson J, Sjögren J, Murugesan V, Hultgårdh-Nilsson A, Cidad P, Hellstrand P, Pérez-García MT and Albinsson S (2015) Regulation of smooth muscle dystrophin and synaptopodin 2 by actin polymerization and vascular injury. Arter. Throm Vasc Biol 35:1489-1497.
- Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernández-Peña C, Talavera A, Kichko T, Navia B, Sánchez A, Señarís R, Reeh P, Pérez-García MT, López-López JR, Voets T, Belmonte C, Talavera K, Viana F. (2014) TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins Nat Commun.;5:3125.
- Tajada S, Cidad P, Colinas O, Santana LF, López-López JR, Pérez-García T, (2013). Down-regulation of CaV1.2 channels during hypertension: How fewer CaV1.2 channels allow more Ca2+ into hypertensive arterial smooth muscle. J. Physiol ;591(Pt 24):6175-91
- Tajada S, Cidad P, Moreno-Domínguez A, Pérez-García MT, López-López JR (2012) . High blood pressure associates with the remodeling of inward rectifier K+ channels in mice mesenteric vascular smooth muscle cells. J. Physiol 590(Pt 23):6075-91
- Cidad, P, Jiménez-Pérez L, García-Arribas D, Miguel-Velado E, Tajada S, Ruiz-McDavitt C, López-López JR and Pérez-García MT (2012) Kv1.3 channels can modulate cell proliferation during phenotypic switch by an ion flux-independent mechanism. Arteriosc Thromb Vasc Biol 32(5): 1299-1307
- Miguel-Velado E, Pérez-Carretero FD, Colinas O, Cidad P, Heras M, López-López JR & Pérez-García MT. (2010) Cell-cycle dependent expression of Kv3.4 channels modulates proliferation of human uterine artery SMCs. Cardiovasc Res 86: 383-391. Editorial Comment
- Cidad P, Moreno-Domínguez A, Novensá L, Roqué M, Barquín L, Heras M, Pérez-García MT, López-López JR (2010). Characterization of ion channels involved in the proliferative response of femoral artery smooth muscle cells. Arterios Thromb Vasc Biol 32: 1299-1307. Editorial Comment
- Moreno-Domínguez A, Cidad P, Miguel-Velado E, López-López JR & Pérez-García MT. (2009). De novo expression of Kv6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain. J Physiol 587(Pt 3):625-40.
- Miguel-Velado E, Moreno-Domínguez A, Colinas O, Cidad P, Heras M, Pérez-García MT & López-López JR. (2005). Contribution of Kv Channels to Phenotypic Remodelling of Human Uterine Artery Smooth Muscle Cells. Circ Res 97(12): 1280-7
- Riesco AM, Pérez-García MT, González C & López-López JR. (2001). O2 Modulates Large-Conductance Ca2+-Dependent K+ Channels of Rat Chemoreceptor Cells by a Membrane-Restricted and CO-Sensitive Mechanism. Circ Res 89:430-436.
- Pérez-García MT, López-López JR, Riesco AM, Hoppe UC, Marbán E, González C & Johns DC. (2000). Viral gene transfer of dominant-negative Kv4 construct supresses an O2-sensitive K+ current in chemoreceptor cells. J Neurosci 20(15), 5689-5695.
- Pérez-García MT, López-López JR & González C. (1999). Kvβ1.2 subunit coexpression in HEK cells confers O2 sensitivity to Kv4.2 but not to Shaker channels. J Gen Physiol 113, 897-907.
- Chiamvimonvat N, Pérez-García MT, Ranjan R, Marbán E. & Tomaselli GF. (1996). Depth asymmetries in the pore-lining segments of the sodium channels revealed by cysteine mutagenesis. Neuron 16, 1037-1047.
- Pérez-García MT, Chiamvimonvat N, Marbán E & Tomaselli GF. (1996). Structure of the Na channel pore revealed by cysteine mutagenesis. Proc Nat Acad Sci (U.S.A.) 93, 300-304.
- López-López JR, Shacklock PS, Balke CW, Wier WG (1995) Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science 268(5213) 1042-45
- López-Barneo J, López-López JR, Ureña J, González C (1988). Chemotrasnduction in the carotid body: K+current modulated by pO2 in typeI chemorreceptor cells. Science 241(4865) 580-2.
Main strengths
Our research philosophy We use a problem-based approach, so that the questions raised lead to the development of new techniques to answer them. This approach has enriched our research (making it more thoughtful, focused and consistent, with a multidisciplinary standpoint), our group (with a large cluster of state of the art techniques ongoing) and the formative process of our students, that could obtain a solid basic formation. The impact of our research. Our group has a good level of publications, many of them relevant contributions to the field, and most of them (>90% of the total in the first quartile of the JCR) Our research funds. We have been able to attract public funds for research since starting independently in 2001, we have been financed sequentially by 5 consecutive projects of the Spanish Government (BFU, National Plan), In addition, we have obtained other resources for personal and research form the ISCIII and the regional government. Our students. We are very proud of them. We have contributed to the formative process of several students, many of them are at the present moment working in leading laboratories worldwide. The group has wide experience in formative activities related to PhD program, participating in the Master and PhD program, and supervising graduate and summer students as well. Our collaborations We have many stable collaboration with internationally recognized groups in the field of ion channels and vascular smooth muscle funcion. We have collaborators and friends all over the world, and overall a well established position among our colleagues. In adittion, we have also been working in the frame of the ISCIII networks for a long time. This experience has fostered our research with many translational applications and network activities with several basic, clinical and epidemiological groups that are still active.The techniques we master We are well-recognized experts in the use of electophysiological  techniques for the study of ion channels, from current-clamp to voltage-clamp experiments in isolated cells. We have the equipment to obtain whole-cell and single-channel currents, to explore these currents in combination with microfluorometric determinations of intracellular ion concentrations and to correlate these currents with gene expression by performing single-cell PCR after functional studies. We complete the single-cell studies with studies in whole arteries using pressure miography to explore integrated vascular responses.
techniques for the study of ion channels, from current-clamp to voltage-clamp experiments in isolated cells. We have the equipment to obtain whole-cell and single-channel currents, to explore these currents in combination with microfluorometric determinations of intracellular ion concentrations and to correlate these currents with gene expression by performing single-cell PCR after functional studies. We complete the single-cell studies with studies in whole arteries using pressure miography to explore integrated vascular responses.
Former group members
- Alba Santos-Hipólito (Master student) 2013-2015
- Laura Jiménez-Pérez (PhD student) 2009-2015
- Yamila Carmona (PhD Student) Erasmus mundus movility program, 2013
- Chrsitian Ruiz-McDavitt (PhD student) 2007-2013
- Sendoa Tajada Esteban (PhD Student) 2007-2013
- Agustín Asuaje (PhD Student) Erasmus mundus movility program 2012-2013
- Francisco Daniel Pérez Carretero (Master student) 2010-2012
- Daniel García Arribas (MD) 2010-2013
- Dr Eduardo Miguel Velado (postdoctoral researcher) 2003-2012
- Alejandro Moreno Domínguez (PhD Student) 2004-2009
- Olaia Colinas Miranda (PhD student) 2002-2009

Active and ongoin colaborations of the group:
International colaborations (joint projects and/or papers)- Dr Karel Talavera, KU Leuven, Belgica
- Dr Colin Nichols, Washington University, St Louis, Missouri, USA
- Dr Sebastian Albisson, Lund Univerity, Sweden
- Dr Iain Greenwood, St George Univesity London, UK
- Dr Fernando Santana, University of California at Davis, USA
- Dr William C Cole, Calgary University, Canada
- Dra Mercé Roqué Moreno, IDIBAPs y Hospital Clinic, Barcelona
- Dr Jaume Marrugat, IMIM, Barcelona
- Dr Oscar Casis, UPV/EHU, Vitoria
- Dr Diego Álvarez de la Rosa, U de la Laguna, Tenerife
- Dr Manuel Navedo, University of California at Davis, USA
- Dr Jason X Yuan, University of Arizona at Tucson, USA
- Dr Maria F Gómez, Lund University, Sweden
- Dr Luis A Pardo, Max-Plank Institute for Experimental Medicine, Gottingen, Germany










